Scientists at Stanford and the SLAC National Accelerator Laboratory recently captured high-resolution video that shows proteins assembling into a crystal, molecule by molecule. The team focused on imaging the surface layer of Caulobacter crescentus, a bacterium that lives in lakes. The surface layer (S-layer) is the outermost layer of the cell.
“The S-layer itself is not fully understood. We wanted to understand the process of its formation and why the S-layer is so strong,” said chemistry professor W.E. Moerner, who shared the 2014 Nobel Prize in Chemistry.
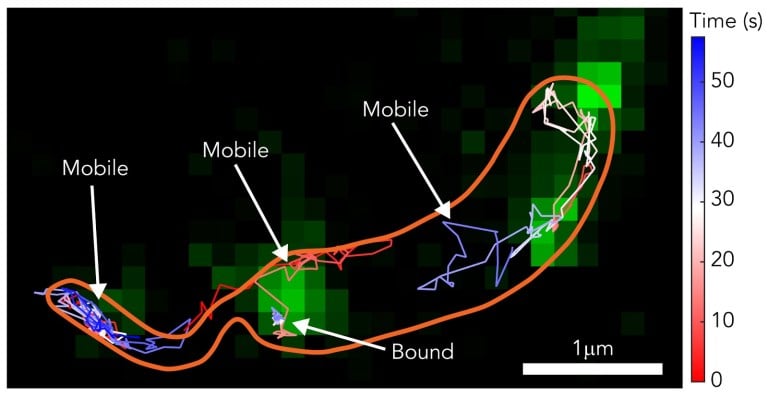
Using single-molecule tracking and high-resolution microscopy, researchers were able to see how the S-layer forms and develops. This knowledge is useful in learning about the defense system of the cell.
New possibilities
The S-layer is one of the strongest parts of a Caulobacter crescentus cell. It is the first to make contact with any pathogens.
“The potential applications are enormous,” said photon science and structural biology professor Soichi Wakatsuki Ph.D. ’91. “The S-Layers of cells can protect themselves from external attacks. Understanding how they do this will help us learn how to attack them cleverly,”
With the help of the video, researchers and drug developers can see how to break down the surface layer and successfully attack a cell. Further, researchers can create drugs to prevent the S-layer from even developing.
“Our research is incremental,” said co-lead researcher Jonathan Herrman Ph.D. ’19. “We are not going to solve anything overnight. But this gives us powerful molecular insight that is the basis for new treatment and new tech.”
With this new knowledge, drug developers can focus on how to weaken the cell’s main defense system and kill it.
Research Process
According to the researchers, experiment design was critical, and they spent much time ensuring the experiment would yield accurate results. The researchers also had to figure out a method for properly analyzing the results.
“The exciting part of it is the actual design of the experiment,” Herrman said. “Science always has its ups and downs. There were long periods where nothing worked and then periods were everything works. You have to be okay with failure.”
In the study, researchers used two methods to properly view the formation of the S-layer. They used Stimulated Electron Depletion (STED) Microscopy to get super-resolution video footage of it forming. STED microscopy uses multiple lasers to overcome light diffraction and maximize image resolution. This imaging technique could also shed light on other biological processes.
“Super-resolution microscopy allows us to look at the whole story: the S-layer, its shape, and its structure down to the nanoscale,” Moerner said.
The researchers used single-molecule tracking to follow certain molecules throughout the formation of the S-layer. They used fluorescent dyes to view these molecules more clearly.
“We were at the nexus of two fields: fluorescence microscopy and structural biology,” said Colin Comerci, co-lead researcher and a Ph.D. candidate in biophysics. “We were trying to probe the structure and understand deeply how the protein was functioning on the surface.”
According to Herrman, the research on formation of the S-layer is similar to “unveiling a tiny corner of the universe. It is about observing at the single-molecule level.”
Contact Manat Kaur at manat ‘at’ object.live.